√ ivdr 243431-Ivdr requirements
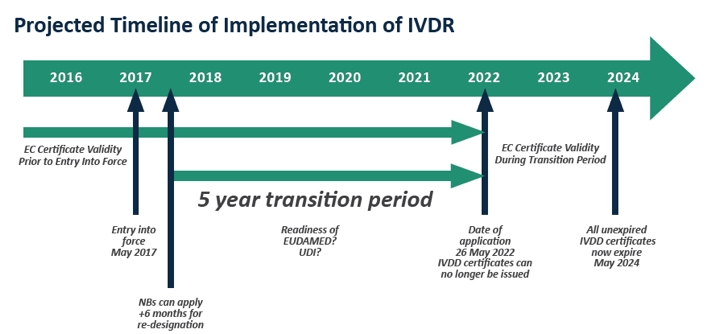
All IVDR Services With 40 years of experience as a provider of testing services for the safety and quality of in vitro diagnostic medical devices (IVD), we are a competent partner for transitioning to the IVDR In addition, we support manufacturers of IVD and medical devices with market access services into the European and international marketsPrinted course presentation, a printed spiral bound book of the IVDR Regulation (EU) 17/746, a short test and a certificate is included for all participants Course Leader/s Emma Axelsson, Senior Quality and Regulatory Consultant AnnaKarin Areskog, Senior Quality and Regulatory ConsultantThe IVDR was put in force by the European Union on May 25, 17 as a replacement for the existing In Vitro Diagnostics Directive (IVDD) The EU has mandated a fiveyear transition period, so IVD manufacturers will have until May 26, 22 to comply with all the new requirements However, the IVDR is regarded as unprecedented in both the number

Ivdr Resource Center Emergo
Ivdr requirements
Ivdr requirements-2 When WAS THE IVDR IMPLEMENTED?March 18, 21 General Status IVDR 14h15h How realistic is the current Date of Application?



Strategic Planning For The In Vitro Diagnostic Regulation Ivdr
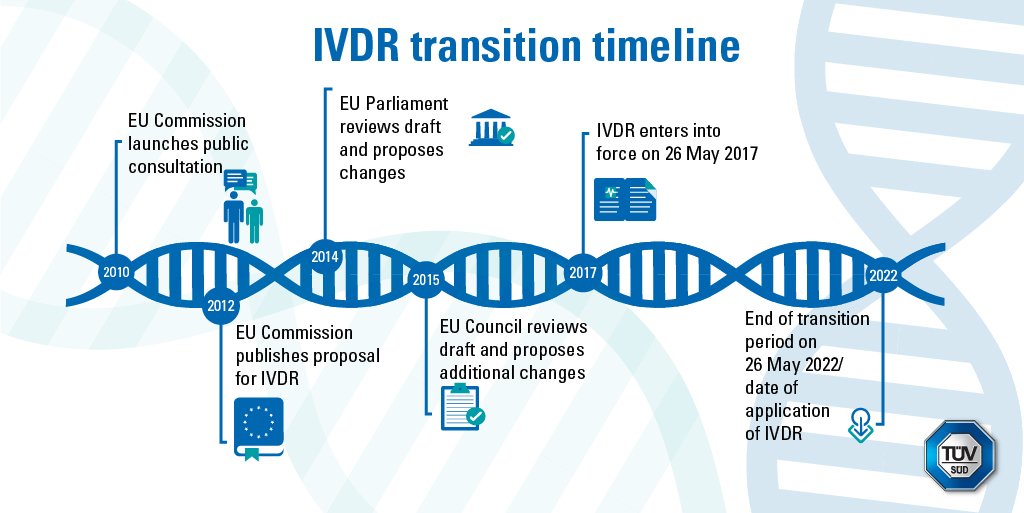
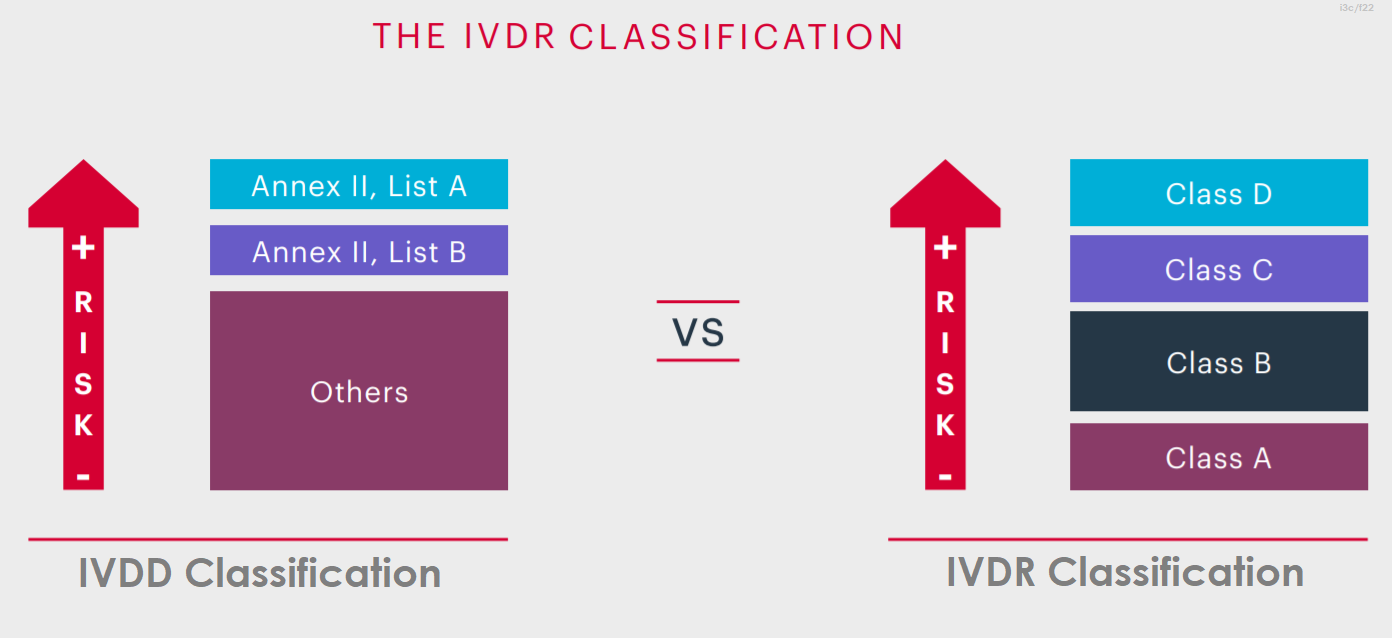
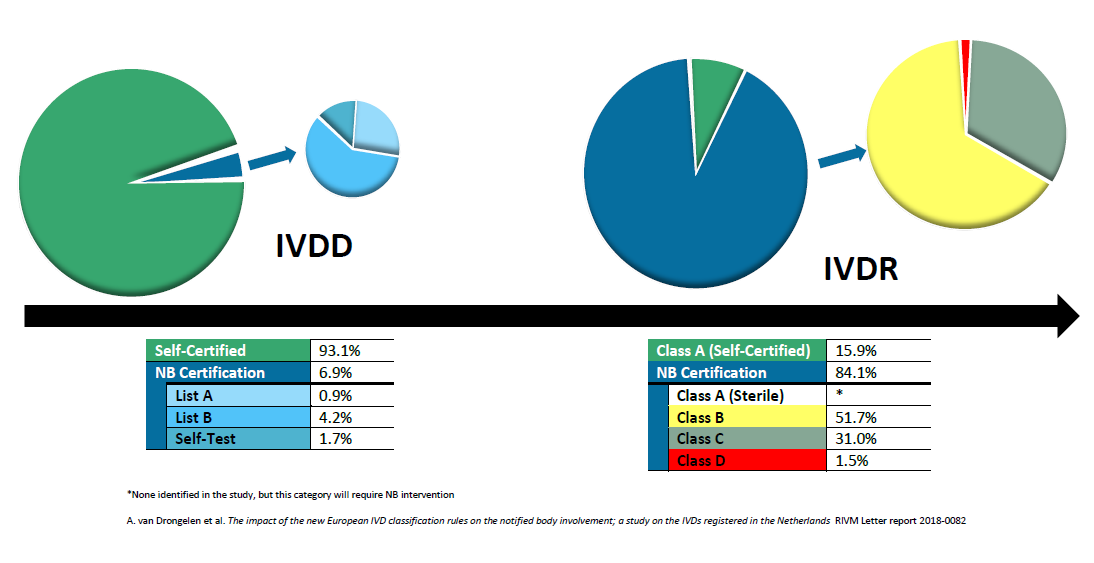
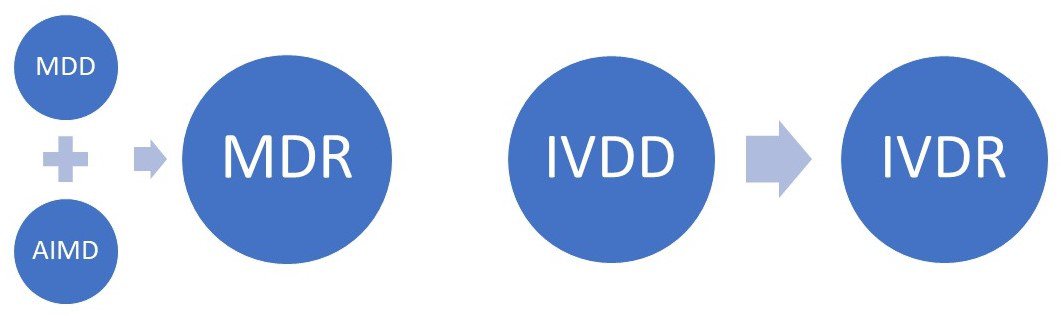
The IVDR uses a riskbased classification system with classes ranging from A (the lowest risk) to D (the highest risk) Under IVDR requirements, a Notified Body must inspect Class ASterile as well as Classes B, C, and D productsAbout IVDR The InVitro Diagnostic Medical Devices Regulation (IVDR) will replace the existing InVitro Diagnostic Medical Devices Directive (IVDD) The IVDR was published in May 17, marking the start of a fiveyear period of transition from the IVDD For further information, please visit EURLex About RochePrinted course presentation, a printed spiral bound book of the IVDR Regulation (EU) 17/746, a short test and a certificate is included for all participants Course Leader/s Emma Axelsson, Senior Quality and Regulatory Consultant AnnaKarin Areskog, Senior Quality and Regulatory Consultant
The new IVDR will be implemented by notified bodies, which have the power to assess if products conform to these regulations before they are placed on the market As of early21, a handful of these have already been designated – including the BSI group in the Netherlands, and TÜV SÜD and Dekra in GermanyOverview Between the COVID19 pandemic and BREXIT negotiations, the rapidly approaching deadlines to comply with the new In Vitro Diagnostics Regulation (IVDR) may have a profound impact on market access to products and, ultimately, patient outcomesEurope's In Vitro Diagnostic Devices Regulation 17/746 (IVDR) will apply in the world's secondlargest medical device market starting in May 22
16h16h30 Break* 16h3017h30 Conformity Assessment Procedures(2) This Regulation aims to ensure the smooth functioning of the internal market as regards in vitro diagnostic medical devices, taking as a base a high level of protection of health for patients and users, and taking into account the small and mediumsized enterprises that are active in this sector At the same time, this Regulation sets high standards of quality and safety for in vitroREGUL ATION (EU) 17/746 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 5 Apr il 17 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision


Tuv Sud The New In Vitro Diagnostic Medical Device Regulation Ivdr Will Replace The Eu S Current Directive On In Vitro Diagnostic Medical Devices 98 79 Ec Find Out More About The New



Eu Medical Device Regulations In Vitro Device Regulations Mdr Ivdr Kallik
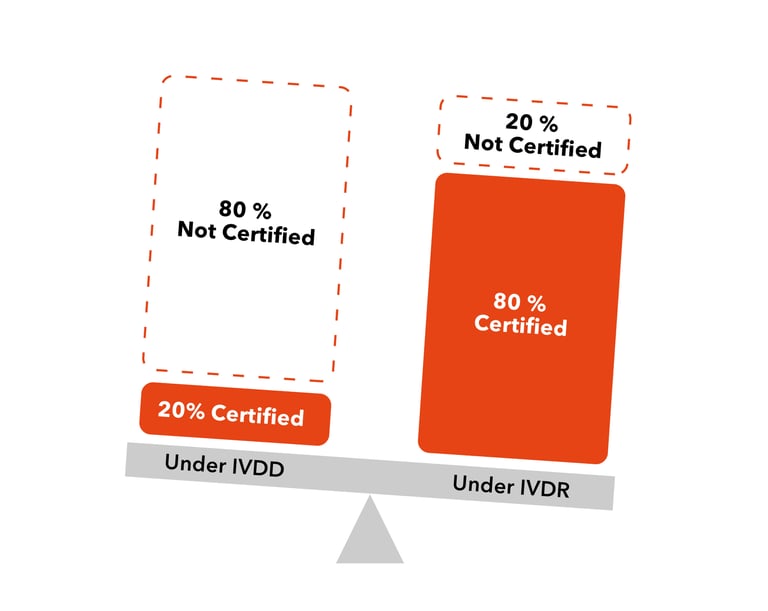
The IVDR will require Notified Body intervention and review for 8090% of IVDs sold in Europe, compared to 10% that were reviewed under the IVDD There are no grandfathering provisions Moderate and highrisk IVDs must be certified to meet the IVDR by May 22 Low risk IVDs have until May 24 but still must meet IVDR QMS requirementsThe IVDR came into force on 25 May 17 Several implementing acts/guidance documents are being issued by the commission and are stipulated to complete some of the requirements to be met 3 When do medical device manufacturers need to comply to the new IVDR?The new IVDR will be implemented by notified bodies, which have the power to assess if products conform to these regulations before they are placed on the market As of early21, a handful of these have already been designated – including the BSI group in the Netherlands, and TÜV SÜD and Dekra in Germany



Ivdr Conformity Assessment Procedures Tuv Sud



Medical Devices Eu Regulations For Mdr And Ivdr Deferred Until May 21
The EU MDR & IVDR Virtual Summit is for you if you are looking to • Meet compliance requirements of EU MDR and IVDR before deadlines and maintain ongoing compliance • Simplify the process of finding a Notified Body and understand how they will conduct audits under new regulations • Meet new technical documentation requirementsThe IVDR was put in force by the European Union on May 25, 17 as a replacement for the existing In Vitro Diagnostics Directive (IVDD) The EU has mandated a fiveyear transition period, so IVD manufacturers will have until May 26, 22 to comply with all the new requirements However, the IVDR is regarded as unprecedented in both the numberThe IVDR also defines a CDx, for the first time in Europe, creating new challenges for device manufacturers and pharmaceutical companies producing CDx Under the previous directives, CDx could be selfcertified Now, considered a highrisk class C device, CDx requires conformity assessments by a notified body (NB), including increased clinical



Economic Operators Eu Mdr And Ivdr Requirements


Mdr Ivdr Timeline Changes Impact Europe It Group
An early start is advised, given the expected pressure on notified bodies leading up to May 22 However, for many, IVDR raises more questions than answers Over the next three months, we will bring you a series of webinars to help you prepare for the transition, providing you with everything you need to know to get ready for IVDREU MDR and IVDR Guidance Documents A Complete List (Including PDF Download Links) November 18, Last Update February 26, 21 Shown below is a list of EU guidance documents endorsed by the Medical Device Coordination Group (MDCG) These documents are the ones we believe are most relevant to medical device and IVD manufacturersWe planned the IVDR transition of our CEmarked portfolio and allocated an adequate budget as well as the necessary resources to ensure that our ~2600 products (catalogue numbers) are IVDRready in time In the preparation phase, we reviewed the Roche CE IVD portfolio and assigned a classification of our IVD portfolio


Akrn Scientific Consulting Classification Of Ivd Under The Ivdr



Ivdd Ivdr Comparison Essential Safety And Performance Requirements Mdlaw Information Platform On European Medical Device Regulations
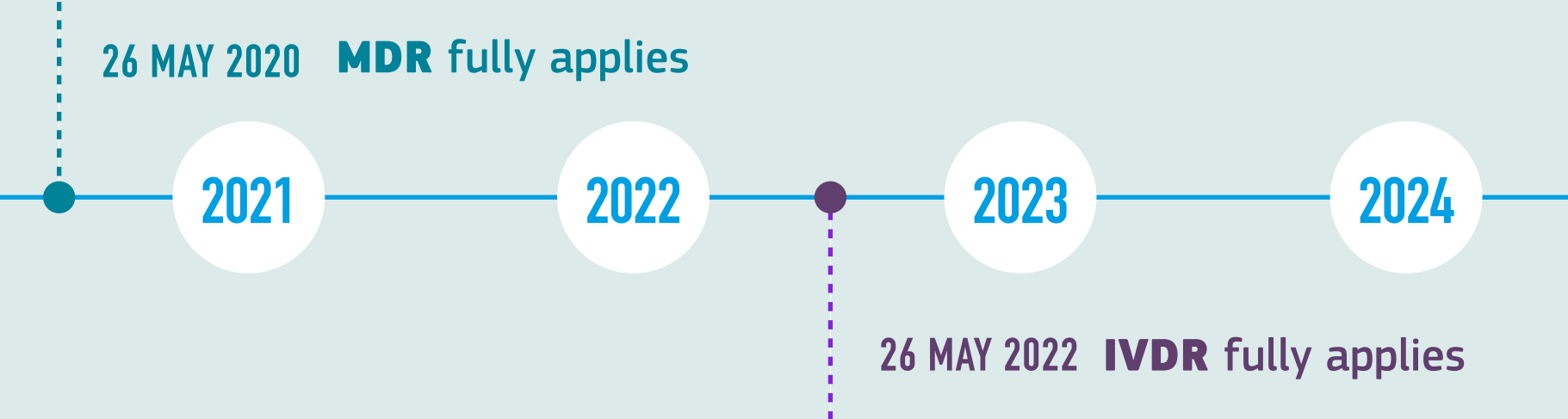
With major changes in legislation affecting device classification and requirements for technical documentation and clinical evidence, companies will need EU Medical Device Regulation (EU MDR) and EU In Vitro Diagnostics Regulation (EU IVDR) training Our courses provide comprehensive instruction on the EU MDR and EU IVDRThe IVDR has brought forward a new drive to establish "a robust, transparent, predictable and sustainable regulatory framework for in vitro diagnostic medical devices which ensures a high level of safety and health whilst supporting innovation"The IVDR came into force on 26 May 17 and has a 5year period of transition This ensures sufficient time for manufacturers and developers to utilise new pathways to market and for suppliers to adapt existing IVDs to comply with new requirements Quality, safety and reliability of IVDs will be improved by the IVDR



Managing Ivdr Regulatory Uncertainty For Localization Multilingual



In Vitro Diagnostic Regulation Ivdr Medical Devices Bsi America
The new EU In Vitro Diagnostics Regulation (EU IVDR) is not radically different from the current IVD Directive (IVDD) That's not to underestimate the amount of work that will be required to switch from the current IVDD to the new EU IVDRAccording to EU IVDR 17/746, Invitro Diagnostic Medical Device is any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, Whether used alone or in combination, intended by the manufacturer to be used invitro for the examination of specimens, including blood and tissue donations, derived fromThe Regulation EU 17/746 (IVDR) replaces the "positive list" approach with new classifications rules, as defined in Annex VIII These classification rules define 4 different levels of risk classes (from the lowest risk class, ie class A, to the highest risk class, ie class D), based on the clinical risk profile of the IVD


Q Tbn And9gcscoehllyatrcaf5sf9mfdacmw1y0tk5ykusuvrr Unxoddyry2 Usqp Cau



Ivdr Classification Ce Marking Operon Strategist
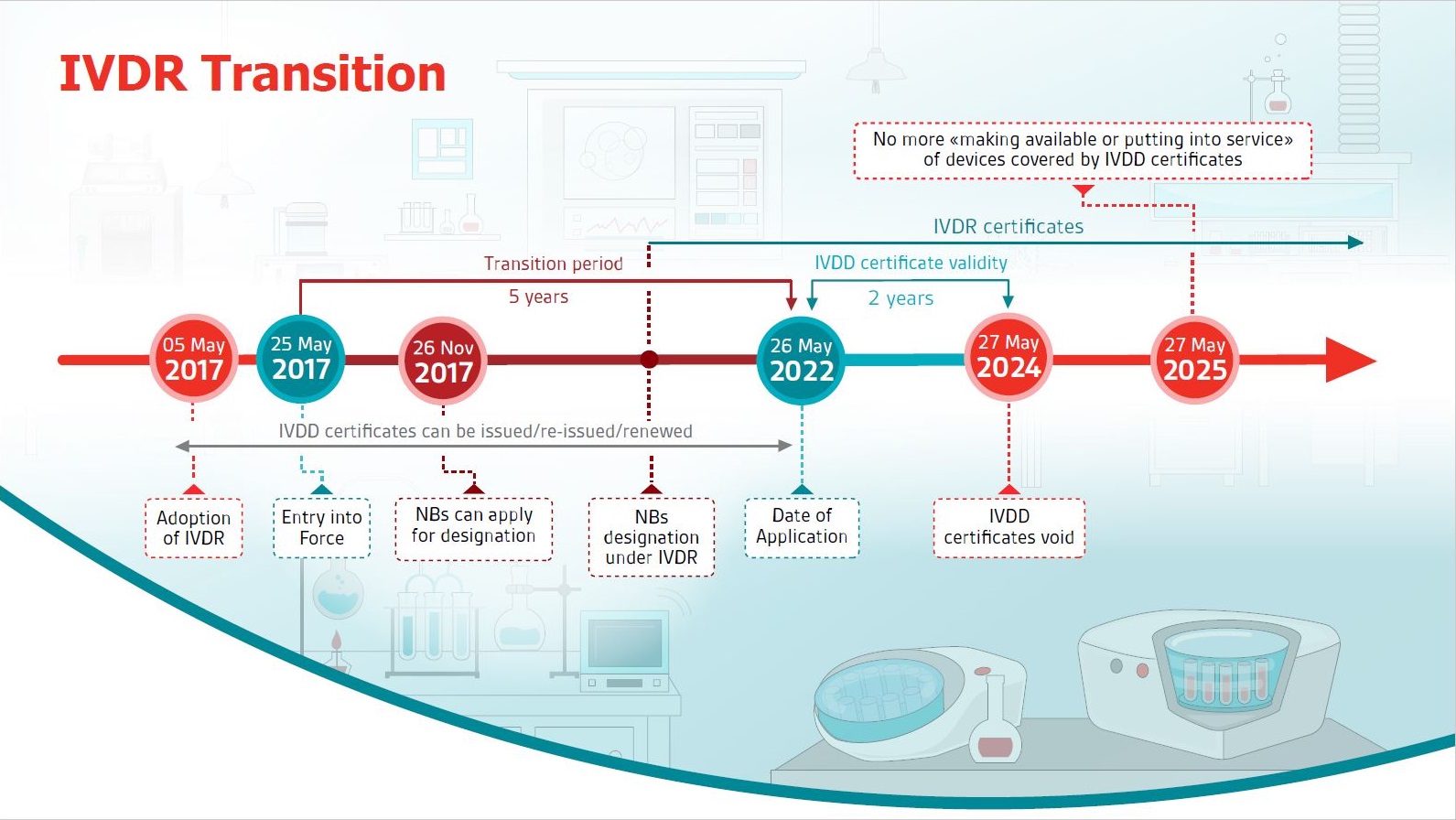
Key IVDR compliance requirements and challenges Creating an actionable roadmap to IVDR compliance is a complex process IVDR introduces new concepts and stricter requirements, including new activities such as clinical data gathering and usability engineering (UE) standards, compared to IVDD, which means manufacturers must now address issues including> IVDR requirements highlighted in yellow are more stringent than the corresponding IVDD requirement, so make sure to check the differences in the wording of both requirements as evidence originally created to satisfy the essential requirements may not be sufficientThe IVDR was officially published on 5 May 17 and came into force on 26 May 17 A transitional period of five years, until 26 May 22, applies to manufacturers of already approved medical products in order to meet the requirements of the IVDR The new regulation focuses on a new classification of IVDs into four risk classes, a more precise



Strategic Planning For The In Vitro Diagnostic Regulation Ivdr



From Ivdd To Ivdr Smart Transition Im Possible Biopro Bw
To conform to Annex I of IVDR 17/746, a GSPR checklist is a mandatory document and is one of the most fundamental preconditions to put any medical device on the EU market MDRG is currently creating an IVDR General Safety & Performance Requirements Checklist that contains a full table of the requirements, along with a list of ApplicableThe week long, online EU MDR & IVDR Virtual Summit will help quality, regulatory and product development professionals understand the new EU medical device regulations and strategic ways for companies to be both successful and competitive in the European market once MDR and IVDR go into effectAll IVDR Services With 40 years of experience as a provider of testing services for the safety and quality of in vitro diagnostic medical devices (IVD), we are a competent partner for transitioning to the IVDR In addition, we support manufacturers of IVD and medical devices with market access services into the European and international markets


Ivdr Classification And Related Rules I3cglobal


In Vitro Diagnostic Regulation Ivdr
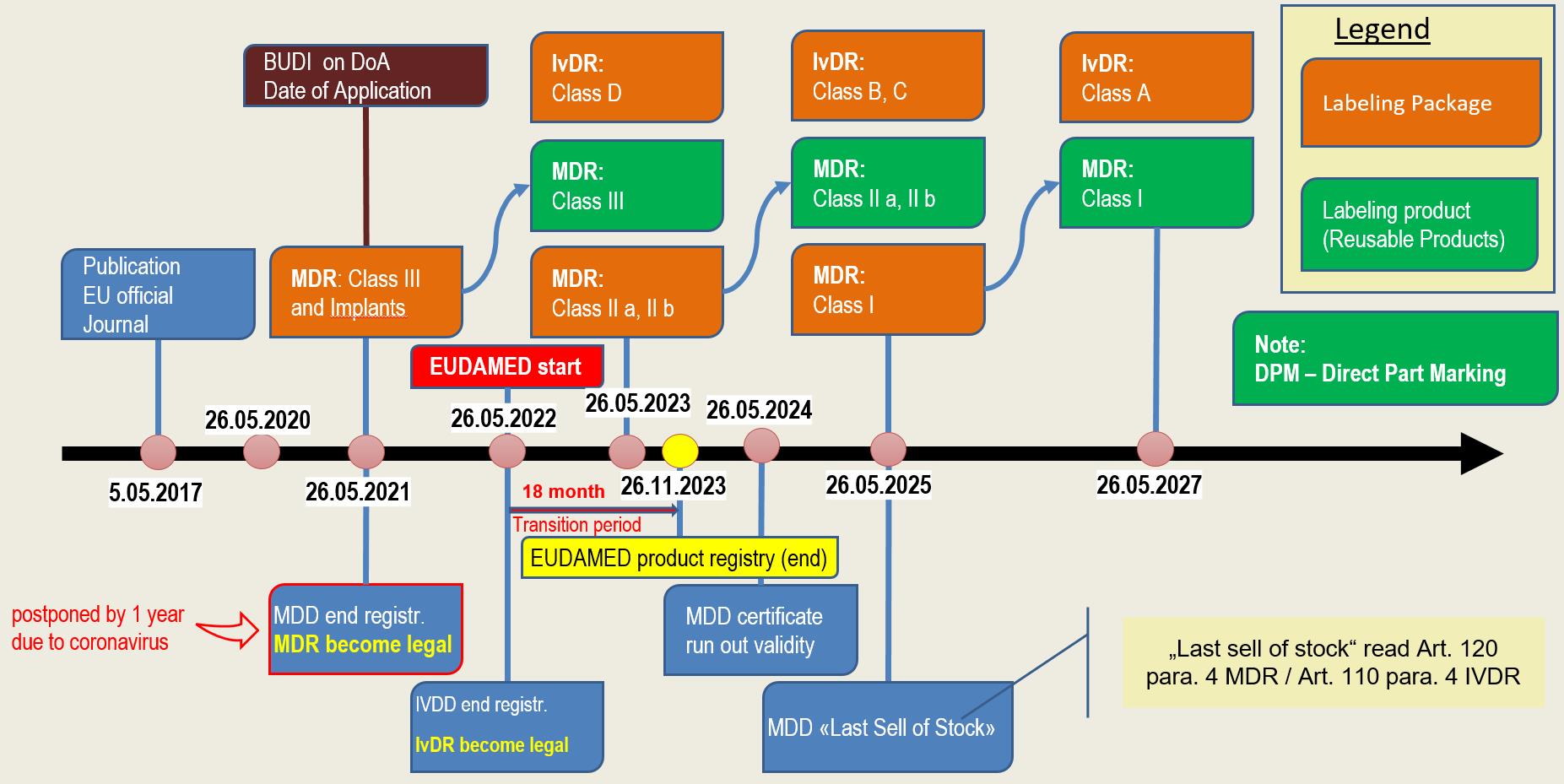
An early start is advised, given the expected pressure on notified bodies leading up to May 22 However, for many, IVDR raises more questions than answers Over the next three months, we will bring you a series of webinars to help you prepare for the transition, providing you with everything you need to know to get ready for IVDRThe two regulations (EU) 17/745 on medical devices (MDR) and (EU) 17/746 on invitro diagnostics (IVDR) came into force on 25 May 17 After a 3year transition period for the MDR (26 May ) and a 5year transition period for the IVDR (26 May 22), the respective regulations become validTo conform to Annex I of IVDR 17/746, a GSPR checklist is a mandatory document and is one of the most fundamental preconditions to put any medical device on the EU market MDRG is currently creating an IVDR General Safety & Performance Requirements Checklist that contains a full table of the requirements, along with a list of Applicable



Medtech Report Eu Mdr And Ivdr Compliance



Identifying The Person Responsible For Compliance Under Mdr Ivdr
Planning for IVDR Compliance IVD manufacturers are familiar with structured systems in the development and management of products However, it is becoming clear that in order to develop and manage a product in accordance with the IVDR, there must be greater emphasis on, and appreciation of, the supporting processes that provide compliance evidenceWe planned the IVDR transition of our CEmarked portfolio and allocated an adequate budget as well as the necessary resources to ensure that our ~2600 products (catalogue numbers) are IVDRready in time In the preparation phase, we reviewed the Roche CE IVD portfolio and assigned a classification of our IVD portfolioThe new EU IVDR corrects this omission but really only brings European importers and distributors into line with already established practices for importers and distributors of medical devices in other geographies That leaves only a couple of requirements in the new EU IVDR which are truly novel



Mdr Ivdr Update



Effect Of New Mdr Ivdr Software Guidance On Remote Patient Monitoring Systems Meddevops
On 13 November, the Medical Device Coordination Group (MDCG) published their long anticipated IVD Classification guidance document for IVDR (MDCG 16) The purpose of the document, "is to provide guidance to manufacturers, notified bodies and health institutions on how to classify an IVD prior to placing it on the market, making available on the market or putting into service in the UnionThis guidance provides information on the EU Regulations for medical devices (MDR) and in vitro diagnostic medical devices (IVDR) From 1 January 21 the Medicines and Healthcare productsOn 13 November, the Medical Device Coordination Group (MDCG) published their long anticipated IVD Classification guidance document for IVDR (MDCG 16) The purpose of the document, "is to provide guidance to manufacturers, notified bodies and health institutions on how to classify an IVD prior to placing it on the market, making available on the market or putting into service in the Union



Ec Names Fifth Notified Body For Ivdr Raps
/tuv-rheinland-ivdr-visual-1-en_core_1_x.png)


In Vitro Diagnostic Medical Device Regulation Ivdr Us Tuv Rheinland
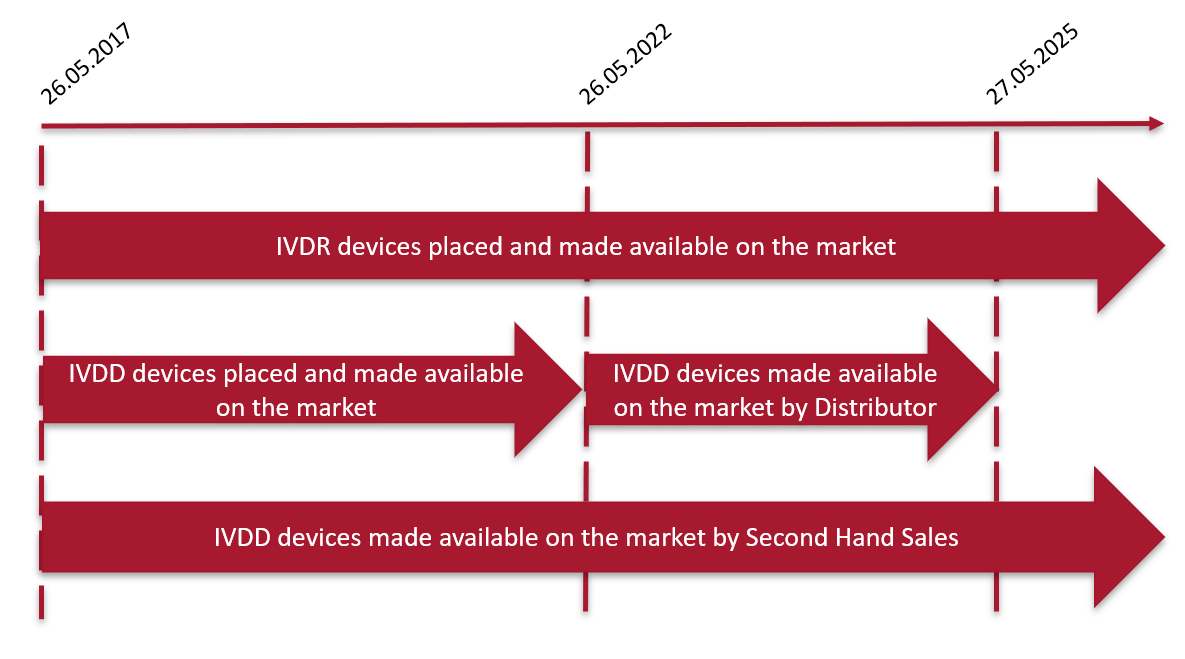
The In Vitro Diagnostic Regulation (IVDR) (EU) 17/746 is the new EU legislation applicable to in vitro diagnostic (IVD) medical devices Entering into force on the 25 May 17 marking the start of a fiveyear transition period for manufacturers and economic operators, the IVDR replaces the EU In Vitro Diagnostics Directive (IVDD) 98/79/ECThe IVDR details the new PMPF requirements, including the dependency on the type and classification of the device being reviewed and how it should be defined within a Quality Management System (QMS) The Post Market Performance Followup (PMPF) is an important element of Post Market Surveillance (PMS)As with the MDR, the IVDR does not provide a "protection of the population" Meaning, all currently approved invitro diagnostics must be reviewed under the terms of the IVDR requirements after the valid IVDD certificate expires and must be then reapproved There is a fiveyear transition period for manufacturers with already approved IVDs



Mdr And Ivdr In 19 Up Or Out Sink Or Swim Medicaldeviceslegal



Are You Ready For Ivdr Implementation Beaufort
The IVDR uses a riskbased classification system with classes ranging from A (the lowest risk) to D (the highest risk) Under IVDR requirements, a Notified Body must inspect Class ASterile as well as Classes B, C, and D products> IVDR requirements highlighted in yellow are more stringent than the corresponding IVDD requirement, so make sure to check the differences in the wording of both requirements as evidence originally created to satisfy the essential requirements may not be sufficientEU IVDR – Regulation (EU) 17/746 Your step by step guide to complying with the European Union's In Vitro Diagnostics Regulation of 17 Step 1 Decide the intended use and classification of the planned IVD device
/tuv-rheinland-ivdr-visual-2-en_core_1_x.png)


In Vitro Diagnostic Medical Device Regulation Ivdr Us Tuv Rheinland



Ivdr Resource Center Emergo
The Regulation EU 17/746 (IVDR) replaces the "positive list" approach with new classifications rules, as defined in Annex VIII These classification rules define 4 different levels of risk classes (from the lowest risk class, ie class A, to the highest risk class, ie class D), based on the clinical risk profile of the IVDThe IVDR also defines a CDx, for the first time in Europe, creating new challenges for device manufacturers and pharmaceutical companies producing CDx Under the previous directives, CDx could be selfcertified Now, considered a highrisk class C device, CDx requires conformity assessments by a notified body (NB), including increased clinicalThe InVitro Diagnostic Medical Devices Regulation (IVDR) will replace the existing InVitro Diagnostic Medical Devices Directive (IVDD) The IVDR was published in May 17, marking the start of a fiveyear period of transition from the IVDD For further information, please visit EURLex


Eu Ivdr Conformity Assessment Options And Pms Requirements


The Blog Mind The Gap How To Navigate The Ivdd Ivdr Transition Part 1
The European Union Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) came into effect as of May 26th, 17 The clock is ticking for current Medical Device manufacturers in the European marketplace to develop and implement strategies to be fully compliant The new In Vitro Diagnostic Regulation (IVDR) is complex and the
.png?width=2400&height=1200&name=medical_device_qms_changes%20(1).png)


Medical Device Qms Changes How To Manage Changes Regarding Eu Mdr Ivdr Iso 16



Mdr Ivdr Implementation Rolling Plan



Update On The Mdr Ivdr Transitional Period Asphalion



Understanding The Mdr Ivdr What Medical Device Companies Need To Know Massdevice



An Mdr And Ivdr Transition Plan Medicaldeviceslegal



Column Ivdr Go With The Flow Medtech Intelligence



Eu Ivdr Requirements For Certification



Mdr And Ivdr Explained By Erik Vollebregt Part 1 Medical Devices Youtube


Combination Products Europe Measures The Challenges Of Implementing Regulatory Changes Mdr Ivdr Pearl Pathways



Ivdr In Vitro Diagnostic Medical Devices Regulation Eu 17 746 Mdi Europa



Tuv Sud Is A Notified Body Under The Ivdr Tuv Sud



What Is The Ivdr Phg Foundation



Ivdr How To Anticipate The Chaos



Ivdr Template Package Eudamed


Mdr Vs Ivdr Comparison Table Regulatory Globe



In Vitro Diagnostic Regulation Ivdr Medical Devices Bsi America


Eu Ivdr Regulatory Changes Overview Of Requirements In 17 746



Expert Article Series On Ivdr Part 1 Introduction To Ivdr Epista Continuously Improving Regulatory Compliance


Ivdr What It Is And Why Early Compliance Matters Advantu
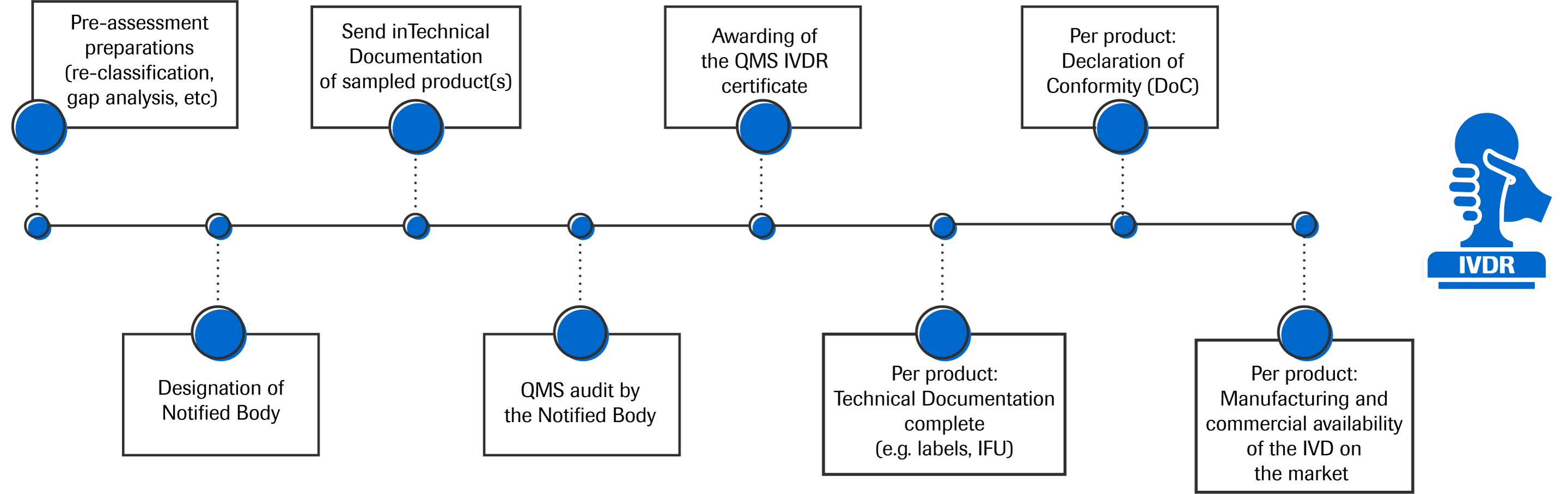


In Vitro Diagnostics Medical Devices Regulation Ivdr Setting The Framework For The European Ivd Market



Understanding The In Vitro Diagnostic Regulation Ivdr Everything You Need To Know
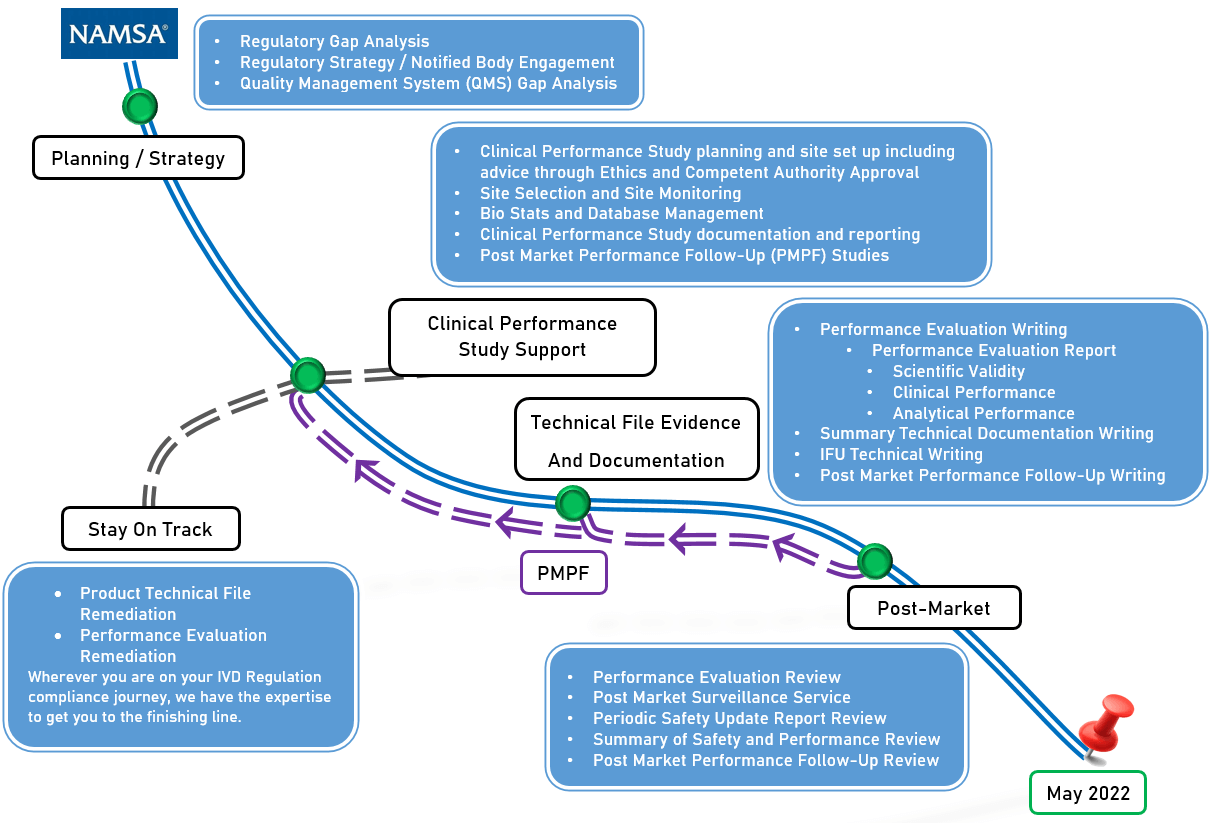


The Ivdr Compliance Roadmap From Start To Finish Namsa



Proclinical Group Mdr Ivdr Guidebook



Eu Regulation On In Vitro Diagnostic Medical Devices What Is Behind It Help



Ivdr E Book Mdlaw Information Platform On European Medical Device Regulations



The Importance Of Intended Purpose And State Of The Art In Implementing Eu S Ivdr



Tips For Reigniting Your Mdr Ivdr Preparation Medical Product Outsourcing



Europe S New In Vitro Diagnostic Regulation Ivdr 17 746


Ivdr Consulting Service Eu In Vitro Diagnostic Regulation Ivdr Training



Implementation Timelines For Eu Mdr And Ivdr Regdesk


Timeline And Transition To The New Medical Device Regulations


5 Tips To Get Ready For The New Eu In Vitro Diagnostic Device Regulation



Eu Mdr Ivdr Implementation Resources Checklists Treximo


Key Aspects Specific Of In Vitro Diagnostics Regulation Ivdr



Treximo Announces Multi Part Mdr Ivdr Readiness Program Treximo



Q A Structuring Pers Under Ivdr



Expert Article Series On Ivdr Part 1 Introduction To Ivdr Epista Continuously Improving Regulatory Compliance


Medical Device White Papers


Akrn Scientific Consulting Classification Of Ivd Under The Ivdr



Eu Mdr And Ivdr Are Live Here Are Some Important Items You Should Know



Expert Article Series On Ivdr Part 1 Introduction To Ivdr Epista Continuously Improving Regulatory Compliance



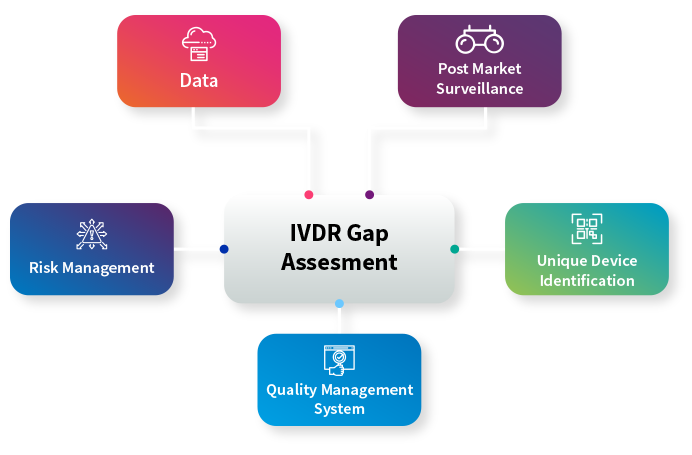
Ivdr Gap Assessment Tool Ivdr Gap Analysis Tool


Q Tbn And9gcqyyruwzcfm8nhfz7nkg04pyoyhicmmvpuibgalptc Usqp Cau



Overcoming New Eu Mdr Ivdr Regulation Challenges



In Vitro Diagnostic Device Regulation Ivdr


Post Market Surveillance As Part Of The Eu Mdr And Ivdr Emma International



New Ivd Regulation Is Coming Are You Ready



Ivdr Technical Documentation 5 Critical Parameters Of Change You Must Understand Namsa



What Is The Ivdr Phg Foundation
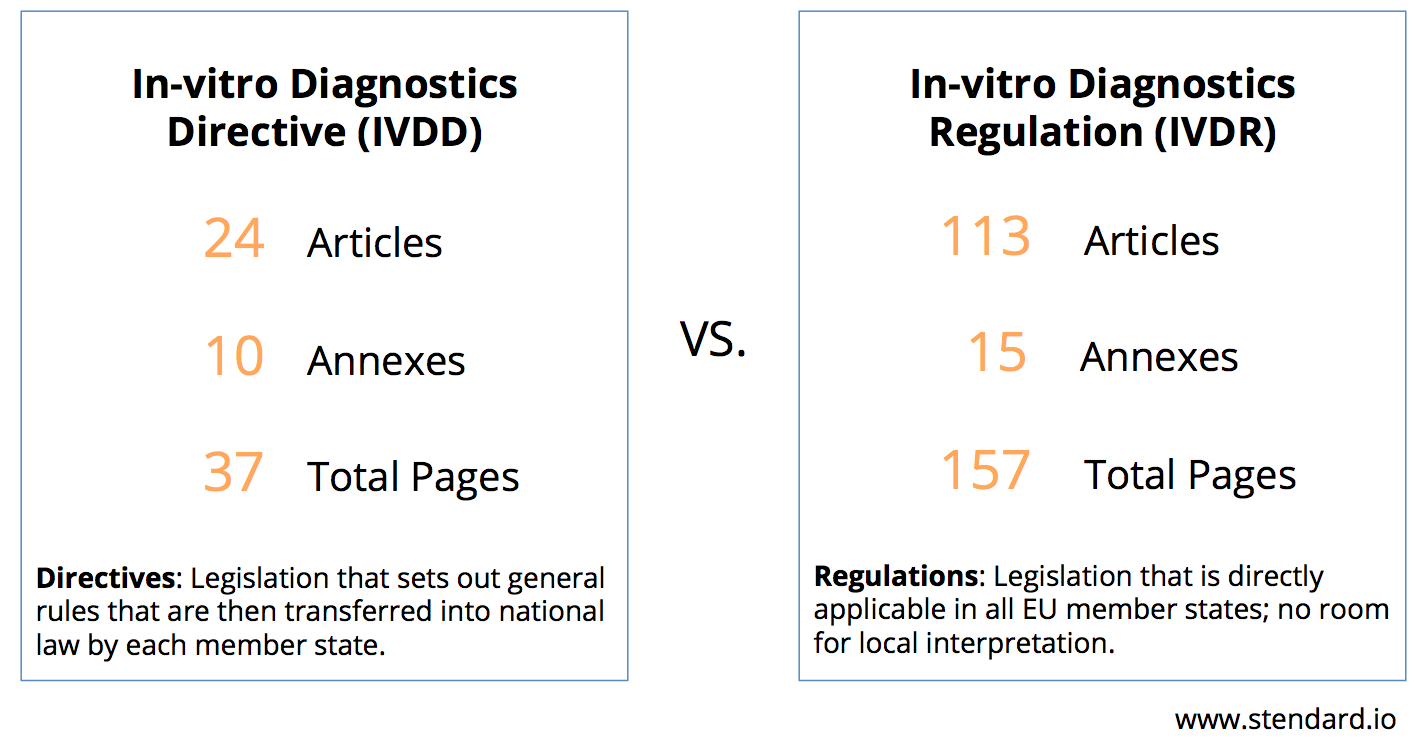


Why Is The New Eu Ivd Regulation Creating A Storm Stendard



Are You Ready For Ivdr Implementation Beaufort
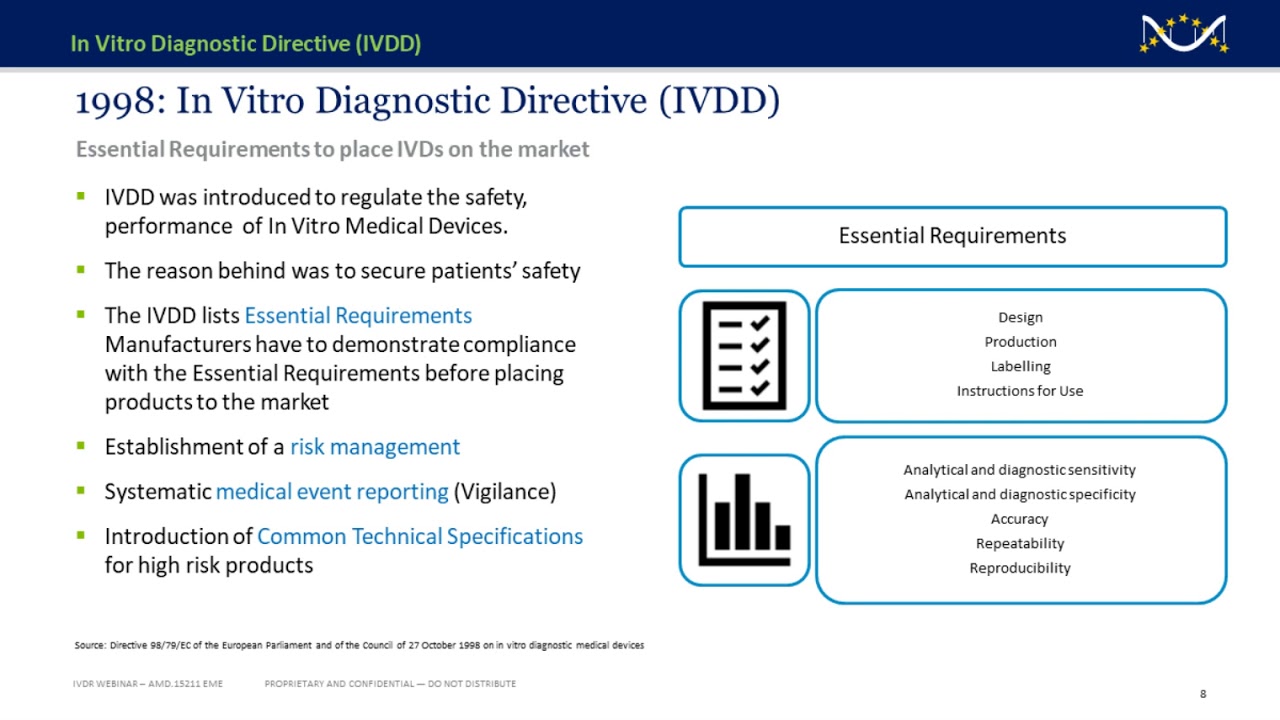


Understanding The In Vitro Diagnostic Regulation Ivdr Everything You Need To Know Youtube



In Vitro Diagnostic Medical Device Regulation Ivdr



How To Build The Right Team For Ivdr Compliance Oxford Global Resources


What Will Pandemic Disruptions Mean For Eu Ivdr Medical Design And Outsourcing
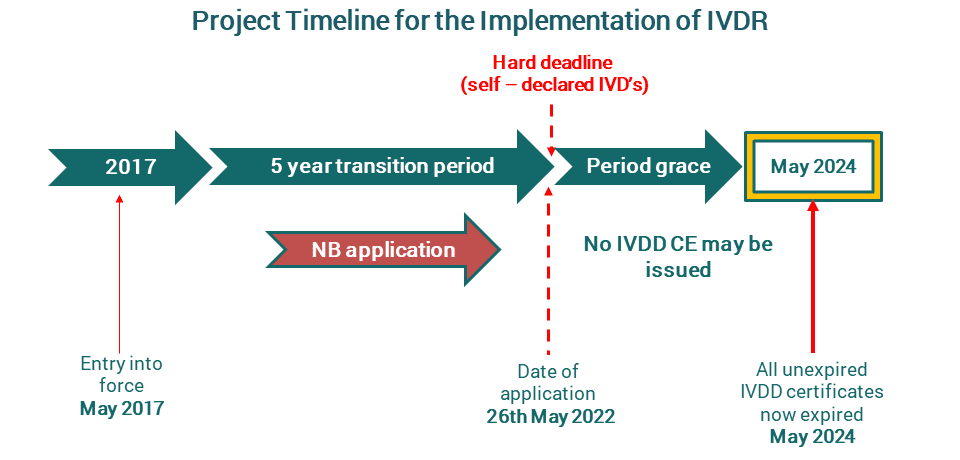


Be Ivdr Ready To Protect Your Lateral Flow Assay And Business



New Legal Obligations Under Mdr And Ivdr



Europe In Vitro Diagnostic Devices Regulation Ivdr Ce Marking Regulatory Process



Mdr And Ivdr Impact In Quality Management Systems Stratesys Consultoria Tecnologica Consultoria Estrategica Sap
.png)


Event Details Raps
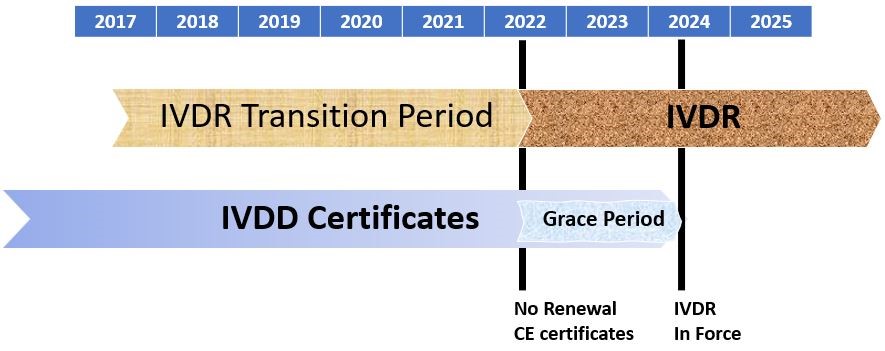


Key Changes In The Regulatory Requirements For In Vitro Diagnostic Devices Marketed In The European Union Under Ivdr 17 746 Criterion Edge


Eu Ivdr In Vitro Diagnostic Regulation 07 746 Key Changes



Webinar Are You Ready For The Ivdr Regulatory Impact And Milestones For Ce Marking


The Ivdr Affects How Genetic Testing Laboratories Can Operate All Over Europe By Ben Liesfeld Limbus News



Standardisation Request For Mdr And Ivdr Refused Now What Medicaldeviceslegal



Eu Companies Ask The Postponement Of The Ivdr Eu 17 746



In Vitro Diagnostic Regulation Ivdr Regulation I3cglobal


Australia The Implementation Of The European Mdr And Ivdr Raises Concerns On The Steady Supply Of Medical Devices And Ivds In Australia Thema Med



In Vitro Diagnostic Regulation Ivdr Bsi


Eu Ivdr Regulatory Changes Overview Of Requirements In 17 746


Ivdr Implementation The Clock Is Ticking Medical Product Outsourcing


The Path To Mdr And Ivdr Compliance For Medical Device Software Med Tech Innovation Latest News For The Medical Device Industry



Transparency Under The New Mdr And Ivdr



Ivdr Classification Ce Marking Operon Strategist Classification Ce Marking Moderation


コメント
コメントを投稿